Have the TGA sold us out to big pharma?
Is the TGA guilty of criminal maleficence? An on-going case study of unethical conduct.
Tacitus, “To show resentment at a reproach is to acknowledge that one may have deserved it.”
Is the Therapeutic Goods Administration (TGA) engaging in fraudulent and unethical behaviour to protect the pharmaceutical companies is it funded by?
For reference – Who is the TGA?
We are Australia's government authority responsible for evaluating, assessing and monitoring products that are defined as therapeutic goods. We regulate medicines, medical devices and biologicals to help Australians stay healthy and safe.
Baseline Accusation
The TGA covered up the deaths of two young Australians, aged 7 and 9, likely caused by mRNA Covid-19 vaccination. By refusing to fulfil their duty to publicly report these deaths and/or obfuscating access to these reports, and in failing to alert the relevant health authorities of potential lethal risk, the TGA have violated and bastardised the basic principles they were designed to protect. Further, knowing that the provisional status of these registrations is dependent on the verifiable safety of the product, and as a result of these findings becoming public, this should have warranted a full suspension of use pending public investigation.
There were ten reports provided in the FOI. Two of these reports had the outcome of “causality.” They were for a 7 and 9 year old boy.
The TGA refused to publish this data on the FOI log. The person who lodged the FOI then took the TGA to the AAT (Administrative Appeals Tribunal) to get the information released, but like every other bureaucracy in the country, the AAT took the side of secrecy rather than transparency.
The deaths haven’t been reported in the fortnightly Covid reports either. This decision has clearly been overruled by the bureaucrats looking to protect the narrative rather than our health.
-Senator Gerard Rennick Website
Motive
Take any and all action to minimise vaccine hesitancy to maintain continued booster uptake as a mechanism to ensure the Australia government purchase more doses, at an increased cost, from suppliers, who in turn provide funding back to the TGA.
***
Supporting evidence
Transgression 1: An attempt to cover up potential wrongdoings
During a Senate Estimates committee on the 16th of February 2023, Senator Gerard Renick asked the head of the TGA, a Mr John Skerritt the following question:
GR: Does myocarditis cause cardiac arrest?
JS: Not usually.
I will ask you review the video yourself so that you can form an understanding of the context.
In my interpretation, it would appear that the Senator is attempting to clarify with the Head of the TGA whether vaccine-induced myocarditis could lead to lethal complications. As in, could the mRNA treatment lead to myocarditis, which in turn causes cardiac arrest and death.
Prior to this exchange, the TGA, through their various publicly available publications have consistently asserted that the vaccine is safe and effective, and that no warning needs to be made for Vaccine-induced cardiac arrest. Nowhere in their documentation have they acknowledged that this connection exists.
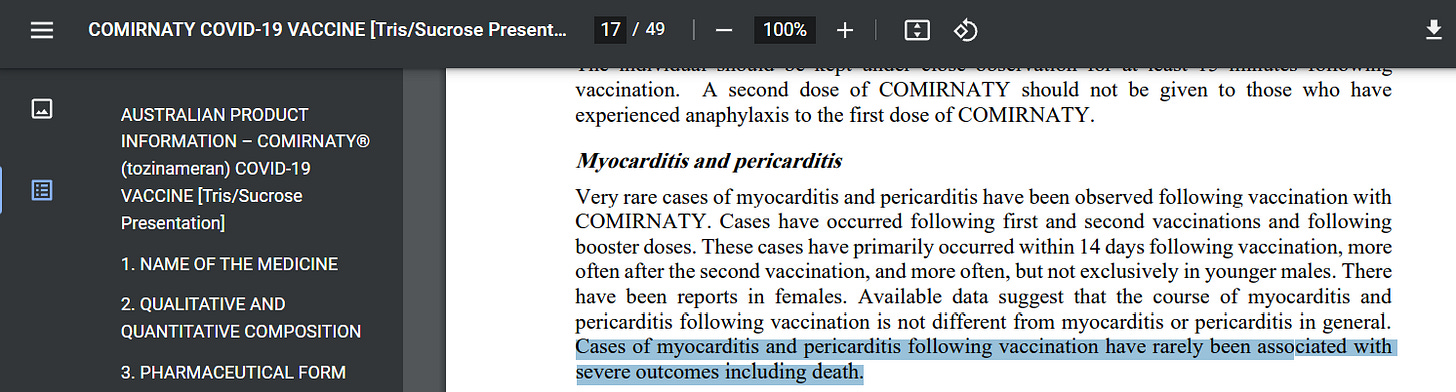
Until now… On the 27th of February 2023, 9 days after this admission in estimates, there was a revision made to the “AUSTRALIAN PRODUCT INFORMATION - COMIRNATY® (tozinameran) COVID-19 - VACCINE [Tris/Sucrose Presentation]” downloadable pdf. Reviewing the available time stamps, this document has remained unchanged since its last revision on the 3rd of December 2021.
When reviewing page 17 we notice the following added revision.
Cases of myocarditis and pericarditis following vaccination have rarely been associated with severe outcomes including death.
The timing of this response is a clear suggestion of a reactionary, and not coincidental, attempt to cover their tracks. In the case of the benefit of the doubt, a window of 11 months has passed since these reports were submitted and therefore ample time has been provided to add this revision if genuine in nature. Only after the discovery was ‘made public’ were the revisions added, suggesting changes would not have been made otherwise.
Transgression 2. Undisclosed changes to safety profile and CMI
When reviewing the COMIRNATY CMI, (a leaflet that contains information on the safe and effective use of a prescription medicine, as well as some non-prescription medicines and some biologicals) we notice that once again subtle revisions have been added.
Previous Version
New version from Feb 2023
You will notice 6 new additions have been made to ‘other side effects’. It will be argued that these are "post-marketing surveillance” (PMS) additions however once again, even factoring in the benefit of the doubt, there are countless journals, studies and news articles that demonstrate a clear connection long before this most recent revision. Once again this suggests a clear indication of reactionary, and not coincidental, attempting to cover their tracks.
It must not be understated that the covert inclusion of heavy menstrual (period) bleeding is an extremely unethical action. Any reaction that may cause issues with reproduction and fertility must be treated with the utmost urgency and as a matter of national emergency. This was a highly reported adverse reaction immediately after the roll out begun, and as such for nearly 2 years, women may have been gaslighted and potentially worse, misdiagnosed, based on this omission.
This must be made public knowledge immediately!!!
Transgression 3. A clear indication of fraudulent misinformation and violation of informed consent.
(Recorded evidence for the record)
Using any search engine; google, duckduckgo, bing etc, and after entering in the following ‘COMIRNATY CMI’, if you were to select the first result in each search engine, you would be directed to the following PDF. Please note that this is not an ad or sponsored link, but the official Australian Government website.
What you will note is that this link will take you to the original CMI that was used prior to the COMIRNATY name change. Note the active ingredient is BNT162b2 [mRNA]. Further note the significantly smaller list of known adverse reactions.
If you were not computer literate and/or not health literate and you were to search for this document online, you would be guided to a document that is nearly 2 years old. This document has been revised multiple times, is significantly out of date and is not clinically accurate.
Once again, and based on the TGA’s history of unethical behaviour, this action cannot be given the benefit of the doubt. Misinformation, and more so harmful disinformation can have dire consequences, and this ‘oversight’ is unforgivable. There are laws in place that stipulate a government is responsible for providing factual and up to date advice regarding adverse reactions. Knowing that all search engines result in the same listing is suggestive that they were informed to use this link directly. As such, someone must be held accountable for not updating this document.
In summation
It is my unqualified opinion that these transgressions are grounds for a full criminal investigation. With minimal effort I was able to discover these horrific violations, so I fear what I would find if a serious investigation were conducted.
This is a government organisation charged with protecting Australians from predatory practises and unsafe pharmaceutical products, and the evidence suggests that they are no longer capable of fulfilling their duties.